Abstract
The global plastics problem is a trifecta, greatly affecting environment, energy and climate1,2,3,4. Many innovative closed/open-loop plastics recycling or upcycling strategies have been proposed or developed5,6,7,8,9,10,11,12,13,14,15,16, addressing various aspects of the issues underpinning the achievement of a circular economy17,18,19. In this context, reusing mixed-plastics waste presents a particular challenge with no current effective closed-loop solution20. This is because such mixed plastics, especially polar/apolar polymer mixtures, are typically incompatible and phase separate, leading to materials with substantially inferior properties. To address this key barrier, here we introduce a new compatibilization strategy that installs dynamic crosslinkers into several classes of binary, ternary and postconsumer immiscible polymer mixtures in situ. Our combined experimental and modelling studies show that specifically designed classes of dynamic crosslinker can reactivate mixed-plastics chains, represented here by apolar polyolefins and polar polyesters, by compatibilizing them via dynamic formation of graft multiblock copolymers. The resulting in-situ-generated dynamic thermosets exhibit intrinsic reprocessability and enhanced tensile strength and creep resistance relative to virgin plastics. This approach avoids the need for de/reconstruction and thus potentially provides an alternative, facile route towards the recovery of the endowed energy and materials value of individual plastics.
This is a preview of subscription content,access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 per month
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Get just this article for as long as you need it
$39.95
Prices may be subject to local taxes which are calculated during checkout
Data availability
The data that support the finding of this study are present in the paper and/or theSupplementary Informationand are available from the corresponding authors on request.
References
Nicholson, S. R., Rorrer, N. A., Carpenter, A. C. & Beckham, G. T. Manufacturing energy and greenhouse gas emissions associated with plastics consumption.Joule5, 673–686 (2021).
Borrelle, S. B. et al. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution.Science369, 1515–1518 (2020).
Geyer, R., Jambeck, J. R. & Law, K. L. Production, use, and fate of all plastics ever made.Sci. Adv.3, e1700782 (2017).
The new plastics economy: rethinking the future of plastics. Ellen MacArthur Foundationhttps://ellenmacarthurfoundation.org/the-new-plastics-economy-rethinking-the-future-of-plastics(2016).
Sullivan, K. P. et al. Mixed plastics waste valorization through tandem chemical oxidation and biological funneling.Science378, 207–211 (2022).
Ellis, L. D. et al. Chemical and biological catalysis for plastics recycling and upcycling.Nat. Catal.4, 539–556 (2021).
Häußler, M., Eck, M., Rothauer, D. & Mecking, S. Closed-loop recycling of polyethylene-like materials.Nature590, 423–427 (2021).
Abel, B. A., Snyder, R. L. & Coates, G. W. Chemically recyclable thermoplastics from reversible-deactivation polymerization of cyclic acetals.Science373, 783–789 (2021).
Beromi, M. M. et al. Iron-catalysed synthesis and chemical recycling of telechelic 1,3-enchained oligocyclobutanes.Nat. Chem.13, 156–162 (2021).
Zhang, F. et al. Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization.Science370, 437–441 (2021).
Li, X.-L., Clarke, R. W., Jiang, J.-Y., Xu, T.-Q. & Chen, E. Y.-X. A circular polyester platform based on simple gem-disubstituted valerolactones.Nat. Chem.15, 278–285(2023).
Rorrer, N. A. et al. Combining reclaimed PET with bio-based monomers enables plastics upcycling.Joule3, 1006–1027 (2019).
Zhu, J.-B., Watson, E. M., Tang, J. & Chen, E. Y.-X. A synthetic polymer system with repeatable chemical recyclability.Science360, 398–403 (2018).
拉希米,a &加西亚,j . m .化学recycling of waste plastics for new materials production.Nat. Rev. Chem.1, 0046 (2017).
Shi, C. et al. Design principles for intrinsically circular polymers with tunable properties.Chem7, 2896–2912 (2021).
Korley, L. T. J., Epps, T. H. III, Helms, B. A. & Ryan, A. J. Toward polymer upcycling – adding value and tackling circularity.Science373, 66–69 (2021).
Coates, G. W. & Getzler, Y. D. Y. L. Chemical recycling to monomer for an ideal, circular polymer economy.Nat. Rev. Mater.5, 501–516 (2020).
Sheldon, R. A. & Norton, M. Green chemistry and the plastic pollution challenge: towards a circular economy.Green Chem.22, 6310–6322 (2020).
Hong, M. & Chen, E. Y.-X. Chemically recyclable polymers: a circular economy approach to sustainability.Green Chem.19, 3692–3706 (2017).
Jehanno, C. et al. Critical advances and future opportunities in upcycling commodity polymers.Nature603, 803–814 (2022).
米ontarnal, D., Capelot, M., Tournilhac, F. & Leibler, L. Silica-like malleable materials from permanent organic networks.Science334, 965–968 (2011).
Rottger, M. et al. High-performance vitrimers from commodity thermoplastics through dioxaborolane metathesis.Science356, 62–65 (2017).
Simhadri, C. et al. Flexible polyfluorinated bis-diazirines as molecular adhesives.Chem. Sci.12, 4147–4153 (2021).
Lepage, M. L. et al. A broadly applicable cross-linker for aliphatic polymers containing C–H bonds.Science366, 875–878 (2019).
Flory, P. J.Principles of Polymer Chemistry(Cornell Univ. Press, 1942).
Bowman, C. N. & Kloxin, C. J. Covalent adaptable networks: reversible bond structures incorporated in polymer networks.Angew. Chem. Int. Ed. Engl.51, 4272–4274 (2021).
Scheutz, G. M., Lessard, J. J., Sims, M. B. & Sumerlin, B. S. Adaptable crosslinks in polymeric materials: resolving the intersection of thermoplastics and thermosets.J. Am. Chem. Soc.141, 16181–16196 (2019).
Fortman, D. J. et al. Approaches to sustainable and continually recyclable cross-linked polymers.ACS Sustain. Chem. Eng.6, 11145–11159 (2018).
Denissen, W., Winne, J. M. & Du Prez, F. E. Vitrimers: permanent organic networks with glass-like fluidity.Chem. Sci.7, 30–38 (2016).
Kloxin, C. J., Scott, T. F., Adzima, B. J. & Bowman, C. N. Covalent adaptable networks (CANs): a unique paradigm in cross-linked polymers.米acromolecules43, 2643–2653 (2010).
Worrell, B. T. et al. A user’s guide to the thiol-thioester exchange in organic media.Polym. Chem.9, 4523–4534 (2018).
Zhang, Q., Qu, D. H., Feringa, B. L. & Tian, H. Disulfide-mediated reversible polymerization toward intrinsically dynamic smart materials.J. Am. Chem. Soc.144, 2022–2033 (2022).
Clarke, R. W., McGraw, M. L., Newell, B. S. & Chen, E. Y.-X. Thermomechanical activation achieving orthogonal work/healing conditions in nanostructured tri-block copolymer thermosets.Cell Rep. Phys. Sci.2, 100483 (2021).
Tretbar, C. A., Neal, J. A. & Guan, Z. Direct silyl ether metathesis for vitrimers with exceptional thermal stability.J. Am. Chem. Soc.141, 16595–16599 (2019).
吉田,T,吉泽章,F。,伊藤,Matsunaga T M. & Watanabe, M. Prediction of fire and explosion hazards of reactive chemicals (Part 1). Estimation of explosive properties of self-reactive chemicals from SC-DSC data.Kogyo Kayaku48, 311–316 (1987).
米usolino, S. F., Pei, Z., Bi, L., DiLabio, G. A. & Wulff, J. E. Structure-function relationships in aryl diazirines reveal optimal design features to maximize C-H insertion.Chem. Sci.12, 12138–12148 (2021).
Chee, G.-L., Yalowich, J. C., Bodner, A., Wu, X. & Hasinoff, B. B. A diazirine-based photoaffinity etoposide probe for labeling topoisomerase II.Bioorg. Med. Chem.18, 830–838 (2010).
Kremer, K. & Grest, G. S. Dynamics of entangled linear polymer melts: a molecular‐dynamics simulation.J. Chem. Phys.92, 5057–5086 (1990).
Self, J. L. et al. Linear, graft, and beyond: multiblock copolymers as next-generation compatibilizers.JACS Au2, 310–321 (2022).
Nomura, K. et al. Multiblock copolymers for recycling polyethylene-poly(ethylene terephthalate) mixed waste.米acromolecules12, 9726–9735 (2020).
Chen, D., Wang, H. & Li, Y. Reactive compatibilization: formation of double-grafted copolymers by in situ binary grafting and their compatibilization effect.米acromolecules9, 33091–33099 (2017).
米acosko, C. W. et al. Compatibilizers for melt blending: premade block copolymers.米acromolecules29, 5990–5998 (1996).
Grest, G. S., Lacasse, M., Kremer, K. & Gupta, A. M. Efficient continuum model for simulating polymer blends and copolymers.J. Chem. Phys.105, 10583–10594 (1996).
米eenakshisundaram, V., Hung, J.-H., Patra, T. K. & Simmons, D. S. Designing sequence-specific copolymer compatibilizers using a molecular-dynamics-simulation-based genetic algorithm.米acromolecules50, 1155–1166 (2017).
Estridge, C. E. & Jayaraman, A. Diblock copolymer grafted particles as compatibilizers for immiscible binary homopolymer blends.ACS Macro Lett.4, 155–159 (2015).
Patra, T. K., Loeffler, T. D. & Sankaranarayanan, S. K. R. S. Accelerating copolymer inverse design using Monte Carlo tree search.Nanoscale12, 23653–23662 (2020).
Plimpton, S. Fast parallel algorithms for short-range molecular dynamics.J. Comput. Phys.117, 1–19 (1995).
Acknowledgements
The work done at Colorado State University (CSU) was supported in part by the US Department of Energy, Office of Science, Basic Energy Sciences under award no. DE-SC0022290, and by the US Department of Energy, Office of Energy Efficiency and Renewable Energy, Advanced Manufacturing Office and Bioenergy Technologies Office, performed as part of the BOTTLE Consortium, which includes members from CSU, and funded under contract no. DE-AC36-08GO28308 with the National Renewable Energy Laboratory, operated by Alliance for Sustainable Energy. This research used resources of the Argonne Leadership Computing Facility and Center for Nanoscience Materials, which are DOE Office of Science User Facilities supported under contract no. DE-AC02-06CH11357. Research conducted at Columbia used resources supported by the Office of The Director, National Institutes of Health of the National Institutes of Health under award no. S10OD026749. The work done at IIT Madras was supported in part by the Science and Engineering Research Board (SRG/2020/001045) and the National Supercomputing Mission (DST/NSM/R&D_HPC_Applications/2021/40), Government of India. T.S. thanks the Swiss National Science Foundation for a fellowship. D.R. thanks the Alexander von Humboldt Foundation for a Feodor Lynen Research Fellowship.
Author information
Authors and Affiliations
Contributions
E.Y.-X.C. and T.R. conceived the project and directed research. R.W.C., T.S., K.A.F., D.R., X.Z. and R.J.T. designed and conducted experiments and analysed results. N.V., S.A., T.K.P. and S.K.K. performed MD modelling studies and analysed results. R.W.C. and T.S. wrote the initial manuscript and revised subsequent versions. E.Y.-X.C. edited the initial draft and S.K.K., T.R. and E.Y.-X.C. edited various subsequent versions.
Corresponding authors
Ethics declarations
Competing interests
A patent (US 2022 63/332,197) has been filed by Colorado State University Research Foundation on findings reported here.
Peer review
Peer review information
Naturethanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s noteSpringer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Additional cross-sectional SEM images of LMWPE-PLLA blends.
a, Virgin immiscible LMWPE-PLLA.b. Compatibilized LMWPE-PLLA blend processed with 5% UDC2.c, Uncompatibilized blend processed with 5% USC.d, Uncompatibilized blend processed with 5% MDC.
Extended Data Fig. 2 Additional cross-sectional SEM images of LMWPE-P3HB blends.
a, Virgin immiscible LMWPE-P3HB.b, Compatibilized blend processed with UDC1.c, Compatibilized blend processed with UDC2.d, Compatibilized blend processed with UDC3.e, Uncompatibilized blend processed with USC.f, Uncompatibilized blend processed with MDC.
Extended Data Fig. 3 Additional cross-sectional SEM images of LDPE-PLLA blends.
a, Extruded immiscible virgin LDPE-PLLA blend.b, Compatibilized blend via reactive extrusion with 5% UDC1.c, Compatibilized blend via reactive extrusion with 5% UDC2.d, Compatibilized blend via reactive extrusion with 5% UDC3.e, Extruded immiscible virgin blend of LDPE bag and PLLA cup flakes.f, Compatibilized blend of LDPE bag and PLLA cup flakes via reactive extrusion with 5% UDC3.
Extended Data Fig. 4 Additional cross-sectional SEM images of LDPE-P3HB blends.
a, Extruded immiscible virgin LDPE-P3HB.b, Compatibilized blend via reactive extrusion with 5% UDC1.c, Compatibilized blend via reactive extrusion with 5% UDC2.d, Compatibilized blend via reactive extrusion with 5% UDC3.
Extended Data Fig. 5 Additional cross-sectional SEM images of binary blends.
a, Extruded immiscible virgin LDPE-iPP blend.b, Compatibilized LDPE-iPP blend via reactive extrusion with 5% UDC3.
Extended Data Fig. 6 Additional cross-sectional SEM images of binary blends.
a, Extruded immiscible virgin polystyrene (PS)-PLLA blend.b, Compatibilized PS-PLLA blend via reactive extrusion with 5% UDC3.
Extended Data Fig. 7 Additional sectional SEM images of ternary blends.
a, Low-magnification image of extruded immiscible LDPE-iPP-PLLA ternary blend.b, High-magnification image of extruded immiscible LDPE-iPP-PLLA ternary blend.c, Low-magnification image of compatibilized LDPE-iPP-PLLA ternary blend via reactive extrusion with 5% UDC3.d, High-magnification image of compatibilized LDPE-iPP-PLLA ternary blend via reactive extrusion with 5% UDC3.
Extended Data Fig. 8 SAXS/WAXS profile for LDPE-PLLA blends.
a, Domain-averaged combination SAXS/WAXS curves for crosslinked LDPE-PLLA blends relative to the virgin blend and reference homopolymers.b, Effect of temperatures above the componentTm’s on domain-averaged combination SAXS/WAXS curves for UDC3 compatibilized LDPE-P3HB blend.
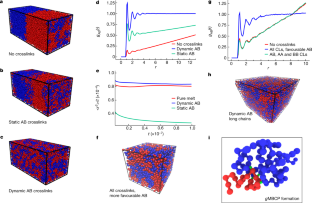
Extended Data Fig. 9 Stability of the interface with all three types of dynamic crosslinks and mixing binary melts with longer chains,n = 100.
a,tal crosslink fraction = 0.1, (AB fraction = 0.01).b,tal crosslink fraction = 0.21, (AB fraction = 0.013). The interface width is stable when all three crosslinks are allowed.c, MD snapshot of a system with all three types of crosslinks allowed showing no mixing.d, Radial distribution functions of three systems – binary melt with no crosslinks (red), and binary melt with all three types of dynamic crosslinks (green) indicate unmixed states, and binary melt with only AB dynamic crosslinks (blue) indicates mixing.
Extended Data Fig. 10 Time evolution of crosslink fraction and stability of the interface with static AB crosslinks.
a, AB crosslink fraction versus time-step for the static (red) and the dynamic (blue) crosslinks. For the static case, the crosslink fraction,f, monotonically increases and reaches 0.16 whereas for the dynamic case it equilibrates at around 0.10.b, The interface width remains stationary at long time showing no further mixing.c, Completely overlappinggAB(r) (att= 23 × 106and 28 × 106time-steps), further showing that the interface is stable and there is no more progress in mixing.
Supplementary information
Supplementary Information
This file contains Supplementary methods, discussion, Figs.1–82, Tables 1–23 and references.
Supplementary Video 1
Video recording of a reactive melt-extrusion of LDPE-PLLA (50/50 wt%) embedded with UDC3 (5 wt%) in a HAAKE Minilab 3 Micro-Compounder (twin-screw extruder) on manual mode. The compatibilized blend extrudate is shown flowing out through the slit die facet.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Clarke, R.W., Sandmeier, T., Franklin, K.A.et al.Dynamic crosslinking compatibilizes immiscible mixed plastics.Nature616, 731–739 (2023). https://doi.org/10.1038/s41586-023-05858-3
Received:
Accepted:
Published:
Issue Date:
DOI:https://doi.org/10.1038/s41586-023-05858-3
Comments
By submitting a comment you agree to abide by ourTermsandCommunity Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
